| You are here: Almanahj Website ⇒ American curriculum ⇒ 8th Grade ⇒ Chemistry ⇒ Term 1 | ||
|---|---|---|
Worksheet about Chemical Bonding | ||
|---|---|---|
| Subject: Chemistry | ||
| 8th Grade | ||
| Term 1 | ||
| Year: 2023/2024 | ||
| Size: 0 | ||
| Number of clicks: 128 | ||
| Publish date:November 07, 2023 | ||
| Added by: Eman | ||
| Last download date: 2024-09-05 13:18:57 | ||
| Updated by: Eman9966 on 2023-11-07 07:46:20 | By: theodor Sridhar Sriram | |
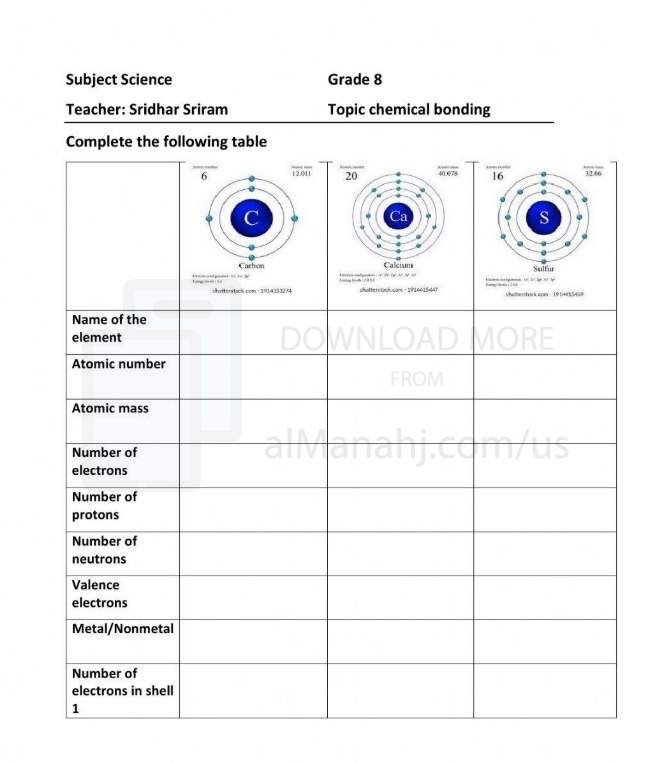
File info: Chemical Bonding:Chemical bonding is the process by which atoms combine to form molecules or compounds. It involves the sharing, transferring, or overlapping of electrons between atoms to achieve a more stable electron configuration. Chemical bonds are responsible for holding atoms together and determining the structure, properties, and reactivity of substances. Key Concepts in Chemical Bonding:1. Valence Electrons: Valence electrons are the outermost electrons in an atom's electron shell. These electrons are involved in chemical bonding and determine the atom's reactivity. The number of valence electrons is generally equal to the group number of the element in the periodic table. 2. Ionic Bonding: Ionic bonding occurs when there is a transfer of electrons between atoms. One atom loses electrons to form a positively charged cation, while another atom gains those electrons to form a negatively charged anion. The resulting attraction between the oppositely charged ions forms an ionic bond. Ionic compounds typically consist of a metal cation and a non-metal anion. 3. Covalent Bonding: Covalent bonding involves the sharing of electrons between atoms. Atoms share one or more pairs of electrons to achieve a more stable electron configuration. This type of bonding is commonly observed in non-metal atoms and results in the formation of molecules. 4. Lewis Dot Structures: Lewis dot structures are diagrams that represent the valence electrons of atoms as dots. These structures provide a visual representation of how electrons are shared or transferred in chemical bonding. 5. Electronegativity: Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. It determines the polarity of a bond. When there is a significant difference in electronegativity between atoms, the bond is polar, and there is an uneven distribution of electron density. In nonpolar covalent bonds, electrons are shared equally. 6. Metallic Bonding: Metallic bonding occurs in metals, where valence electrons are delocalized and form a "sea" of electrons that move freely throughout the metal lattice. This gives metals their characteristic properties, such as malleability, ductility, and conductivity. 7. Intermolecular Forces: Intermolecular forces are the attractive forces between molecules. These forces include dipole-dipole interactions, hydrogen bonding, and London dispersion forces. Intermolecular forces influence the physical properties of substances, such as boiling point, solubility, and viscosity. Applications of Chemical Bonding: Understanding chemical bonding is essential in various areas of chemistry, including: - Predicting and explaining the properties and behavior of substances. - Understanding and designing chemical reactions. - Exploring the structure and function of biological molecules. - Developing new materials with specific properties. - Designing drugs and pharmaceutical compounds. - Analyzing and interpreting spectroscopic data. Overall, chemical bonding provides the foundation for understanding the formation of molecules and compounds. It allows chemists to explain and predict the behavior of substances and enables the development of new materials and technologies. | ||
| File images |
|---|
 |