| You are here: Almanahj Website ⇒ American curriculum ⇒ 12th Grade ⇒ Chemistry ⇒ Term 1 | ||
|---|---|---|
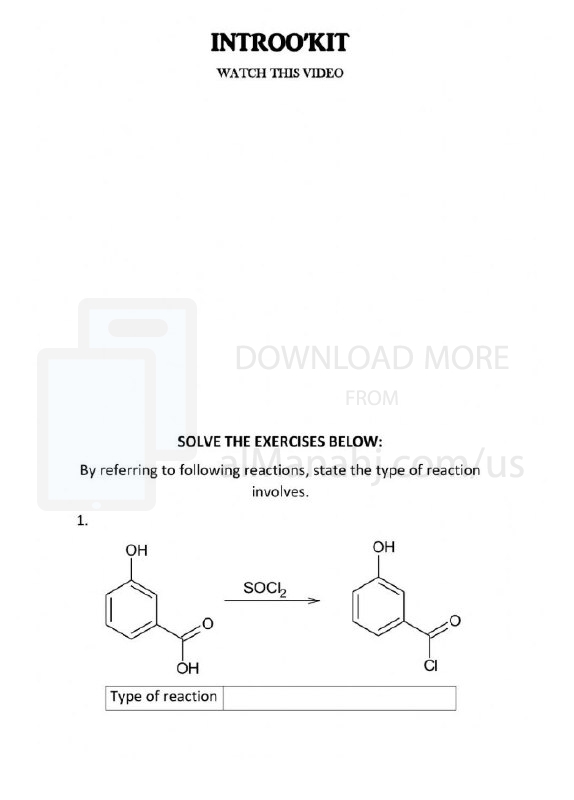
Worksheet about Types of reaction | ||
|---|---|---|
| Subject: Chemistry | ||
| 12th Grade | ||
| Term 1 | ||
| Year: 2023/2024 | ||
| Size: 249.4KB | ||
| Number of clicks: 149 | ||
| Publish date:November 06, 2023 | ||
| Added by: Eman | ||
| Last download date: 2024-09-10 19:14:04 | ||
| Updated by: Eman9966 on 2023-11-06 11:35:46 | By: theodor VINARTI | |
File info: In chemistry, there are several types of reactions that describe how different substances interact and transform into new substances. These reactions are classified based on the changes that occur in the chemical bonds between atoms. Here are some common types of reactions:1. Combination or Synthesis Reactions: In a combination reaction, two or more substances combine to form a single product. The general form of a combination reaction is: A + B → AB For example, the reaction between hydrogen gas (H2) and oxygen gas (O2) to form water (H2O) is a combination reaction: 2 H2 + O2 → 2 H2O 2. Decomposition Reactions: In a decomposition reaction, a single compound breaks down into two or more simpler substances. The general form of a decomposition reaction is: AB → A + B For example, the decomposition of hydrogen peroxide (H2O2) into water (H2O) and oxygen gas (O2) is a decomposition reaction: 2 H2O2 → 2 H2O + O2 3. Displacement or Replacement Reactions: Displacement reactions involve the replacement of one element in a compound with another element. There are two main types of displacement reactions: a. Single Displacement Reactions: In a single displacement reaction, an element reacts with a compound, displacing another element from it. The general form of a single displacement reaction is: A + BC → AC + B For example, the reaction between zinc (Zn) and hydrochloric acid (HCl) to form zinc chloride (ZnCl2) and hydrogen gas (H2) is a single displacement reaction: Zn + 2 HCl → ZnCl2 + H2 b. Double Displacement Reactions: In a double displacement reaction, two compounds exchange ions to form two new compounds. The general form of a double displacement reaction is: AB + CD → AD + CB For example, the reaction between sodium chloride (NaCl) and silver nitrate (AgNO3) to form silver chloride (AgCl) and sodium nitrate (NaNO3) is a double displacement reaction: NaCl + AgNO3 → AgCl + NaNO3 4. Redox Reactions: Redox (reduction-oxidation) reactions involve the transfer of electrons between reactants. There are two main processes in a redox reaction: a. Oxidation: Oxidation is the loss of electrons by a substance, resulting in an increase in its oxidation state. b. Reduction: Reduction is the gain of electrons by a substance, resulting in a decrease in its oxidation state. Redox reactions involve both oxidation and reduction occurring simultaneously. They are essential in various biological and chemical processes and are commonly observed in combustion reactions and reactions involving metals. 5. Acid-Base Reactions: Acid-base reactions involve the transfer of a proton (H+) from an acid to a base. The general form of an acid-base reaction is: Acid + Base → Salt + Water For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) to form sodium chloride (NaCl) and water (H2O) is an acid-base reaction: HCl + NaOH → NaCl + H2O These are just a few examples of the types of reactions in chemistry. The study of reactions and their mechanisms is a central aspect of understanding chemical transformations and the behavior of substances. | ||
| Downloading link Worksheet about Types of reaction |
|---|
|
1699270428.pdf
The file is being prepared for download
|
| File images |
|---|
 |