| You are here: Almanahj Website ⇒ American curriculum ⇒ 9th Grade ⇒ Chemistry ⇒ Term 1 | ||
|---|---|---|
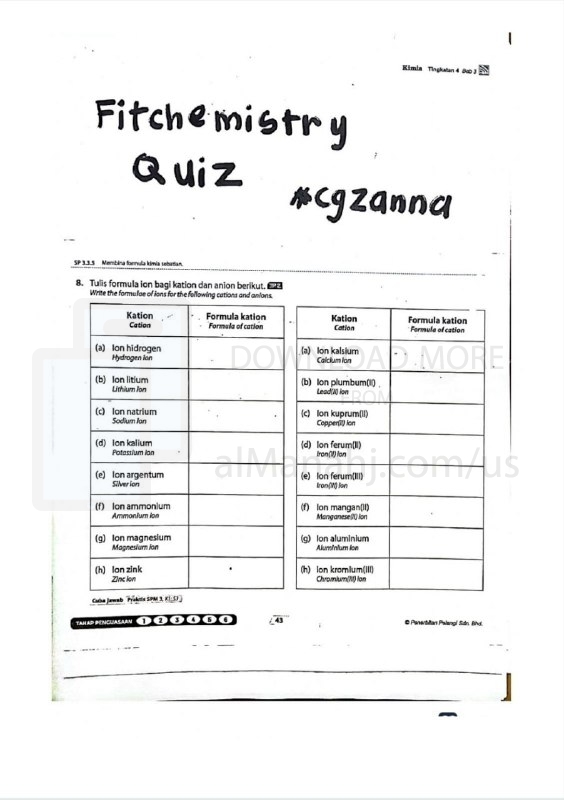
Worksheet about Ionic chemical formula | ||
|---|---|---|
| Subject: Chemistry | ||
| 9th Grade | ||
| Term 1 | ||
| Year: 2023/2024 | ||
| Size: 355.3KB | ||
| Number of clicks: 87 | ||
| Publish date:November 04, 2023 | ||
| Added by: Eman | ||
| Last download date: 2024-07-25 17:56:41 | ||
| Updated by: Eman9966 on 2023-11-04 11:32:21 | By: theodor Ruzanna Abdul Manap | |
| File info: Info An ionic chemical formula is a representation of a compound composed of ions. Ions are charged particles that are formed when atoms gain or lose electrons. In an ionic compound, positively charged ions, known as cations, are attracted to negatively charged ions, called anions, through electrostatic forces. The ionic chemical formula consists of the symbols of the elements present in the compound, along with their respective charges. The cation, which is usually a metal, is written first, followed by the anion, typically a nonmetal or a polyatomic ion. The number of each type of ion is indicated by subscripts, which represent the ratio of ions in the compound. It is important to note that the charges of the ions must balance in the formula to maintain electrical neutrality. For example, in the compound sodium chloride (NaCl), sodium (Na) is a cation with a charge of +1, and chloride (Cl) is an anion with a charge of -1. The formula indicates that there is one sodium ion for every chloride ion, resulting in a neutral compound. Another example is calcium carbonate (CaCO3), where calcium (Ca) is a cation with a charge of +2, carbonate (CO3) is a polyatomic anion with a charge of -2. The formula shows that there is one calcium ion for every carbonate ion, resulting in a neutral compound. Ionic chemical formulas are essential for representing the composition of ionic compounds and understanding their properties, such as their crystal structure, solubility, and conductivity of electricity. They provide a concise and standardized way to communicate the arrangement of ions in a compound. | ||
| Downloading link Worksheet about Ionic chemical formula |
|---|
|
1699097432.pdf
The file is being prepared for download
|
| File images |
|---|
 |